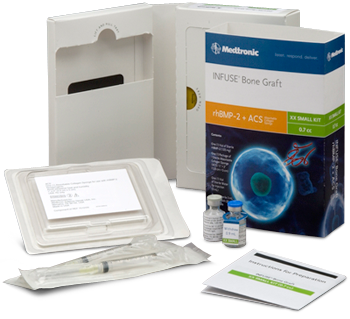
INFUSE Bone Graft promotes natural bone growth to give you long-term implant success.
How INFUSE Bone Graft works
The active component of INFUSE Bone Graft - rhBMP-2- is a recombinantly-produced form of a signaling protein naturally occurring in the human body. This particular protein has been proven to be an important element in the body’s own bone-healing cascade, inducing cells to form biologic bone via osteoinduction.
The rhBMP-2 mechanism of action initiates the body’s own bone-healing cascade:
- INFUSE Bone Graft is implanted in the surgical site.
- Chemotaxis, or attraction of mesenchymal stem cells to the site, occurs.
- Mesenchymal stem cells proliferate, or increase rapidly in number, at the site due to the presence of rhBMP-2, supported by increased angiogenesis at the site.
- Mesenchymal stem cells differentiate, or begin to change from stem cells to osteoblast cells, due to the binding of rhBMP-2 molecules to cell-surface receptors on the stem cells.
- Bone tissue begins to form around the osteoblasts, and it goes on to mineralize and mature as normal anatomic bone does.
- Mechanical forces on the mineralized bone tissue cause the new bone created in the rhBMP-2 implantation site to continuously remodel, just as normal physiologic bone would be expected to respond.
Important information about INFUSE® Bone Graft
 The history of rhBMP-2
The history of rhBMP-2The history of rhBMP-2 extends back decades, providing a wealth of research and studies to support its ability to induce new bone formation.
1965: Dr. Marshall R. Urist discovers that demineralized bone matrix stimulates the formation of new bone tissue in lower-order animal muscle. This led to the isolation of bone morphogenetic proteins (BMPs), the only proteins known to induce new bone formation (osteoinduction).
2002: INFUSE Bone Graft received approval for use in anterior lumbar spine fusion with Medtronic titanium threaded interbody devices.
2004: INFUSE Bone Graft received approval for use in open tibial fractures with intramedullary (IM) nail fixation.
2007: INFUSE Bone Graft received approval for use in sinus augmentation and for localized ridge augmentation for defects associated with extraction sockets.
Winner of the 2008 Prix Galien USA Award for best Biotechnology agent
Reasons to consider INFUSE Bone Graft:
- Eliminates the need for a second bone harvest surgery.
- Regenerates 100% vital, vascular de novo bone with no residual graft material remaining to destabilize bone formation.
- Provides proven, predictable bone formation by combining the bone-generating power of rhBMP-2 with a proven carrier, the absorbable collagen sponge.
- Supported by over 20 years of research and clinical results, including 60 preclinical studies and 5 clinical trials.
Important Information about INFUSE® Bone Graft:
- The potential for prolonged swelling may occur in some (but not all) patients. This is most likely due to the influx of the patient’s own cells and fluids into the treatment site.
- INFUSE Bone Graft has not been studied in patient who are skeletally immature (<18 years of age or no radiographic evidence of epiphyseal closure.)
- INFUSE Bone Graft should not be used in patients with an active infection at the operative site.
- INFUSE Bone Graft should not be used in the vicinity of a resected or extant tumor, in patients with any active malignancy or patients undergoing treatment for malignancy.
- This product has not been tested in pregnant women to determine if it could harm a developing fetus. This product has also not been studied in nursing mothers.
Women of childbearing age should not become pregnant for one year following treatment with the product. Women of childbearing age should be warned of potential risks to a fetus and should discuss other possible treatments with their doctor.
For more information about INFUSE Bone Graft please visit www.infusebonegraft.com.

PMD008414-2.0





.jpg)


.jpg)



.png)








.jpg)



 İmplant
İmplant
 Ortodonti
Ortodonti
 Tranier
Tranier
 Disposable
Disposable
